Indian CAR-T Cancer Therapy: Now At One-tenth Of The Cost.
India is currently producing state-of-the-art CAR-T cancer therapy at a much reduced cost, only one-tenth of the original price.
WFY BUREAU INDIA: The NexCAR19 treatment offers promising prospects for increasing accessibility to this revolutionary category of medication in low- and middle-income nations.
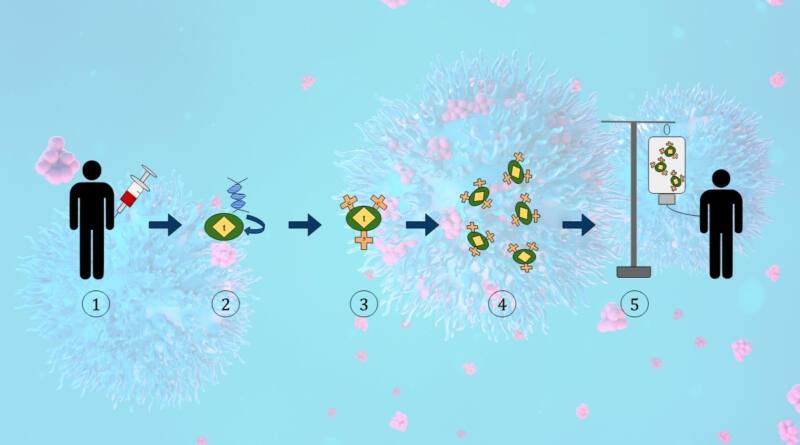
An Indian biotechnology company is producing an indigenous version of chimeric antigen receptor (CAR) T-cell therapy, a cutting-edge cancer medication initially developed in the United States. CAR-T treatments are mostly employed for the treatment of haematological malignancies and have experienced significant growth in recent years. The cost of the Indian CAR-T therapy is only 10% of the price of similar commercial goods accessible worldwide.
The cost of a single administration of NexCAR19, produced by ImmunoACT, based in Mumbai, ranges from $30,000 to $40,000. The initial approval of CAR-T therapy occurred in the United States in 2017. Presently, the cost of commercial CAR-T therapies ranges from $370,000 to $530,000, excluding other expenses such as hospital fees and medications for managing adverse effects. Furthermore, these therapies have demonstrated potential in the treatment of autoimmune disorders and malignant brain tumours.
In October, India’s pharma regulator granted approval for the therapeutic use of NexCAR19 in India. In December, ImmunoACT commenced the provision of the therapy to patients who were willing to pay, and currently, it is treating approximately twenty-four individuals per month in hospitals nationwide.
“It is a realisation of a long-held aspiration,” expresses Alka Dwivedi, an immunologist who played a pivotal role in the creation of NexCAR19 and currently works at the US National Cancer Institute (NCI) in Bethesda, Maryland. Her voice assumes a gentle tone as she recounts witnessing the initial patient’s cancer entering a state of remission. Dwivedi states that these individuals are those who have not had success with any other therapy. “Now they are experiencing healing!”
“The news is highly encouraging,” remarks Renato Cunha, a haematologist at the Grupo Oncoclínicas in São Paulo, Brazil. According to him, the Indian product has the potential to enable the availability of advanced cellular therapies in other low- and middle-income countries. “Hope is the term that I associate with.”
Terry Fry, an immunologist and paediatric oncologist at the University of Colorado Anschutz Medical Campus in Denver, states that the product serves as a reality check for researchers in high-income countries. He has provided guidance to the researchers engaged in establishing ImmunoACT. “Examining the expenses associated with producing CAR-T cells, even in countries like the United States, motivates us all.”
Enormous Requirement
CAR-T therapy entails the extraction of an individual’s blood and the subsequent isolation of immune components called T cells. In a controlled environment, we genetically alter these organisms to produce a specific receptor, known as a CAR, on their outer layer. This facilitates the identification and eradication of cancer cells by immune cells. We then manufacture the manipulated cells in large quantities and reintroduce them into the patient’s body, where they multiply and begin their functions.
The competition to enhance the potency of T cells in combating cancer
There is limited data available on the demand for these therapies in India. However, a study focusing on a particular type of leukaemia revealed that out of every 100,000 individuals, up to 15 are diagnosed with the disease. Among these individuals, half experience a relapse within two years of undergoing treatment, such as chemotherapy, and subsequently opt for palliative care. Nirali Shah, a paediatric oncologist at the NCI and an academic colleague of the researchers at ImmunoACT, emphasises the significant demand for patient care.
NexCAR19 shares similarities with its American competitors, but it also possesses special characteristics that set it apart. This therapy, like four out of the six CAR-T therapies approved by the US Food and Drug Administration (FDA), specifically targets CD19, a marker present in B-cell malignancies. However, mice typically provide the antibody fragment at the end of a chimeric antigen receptor (CAR) in current commercial medicines. This poses a limitation to its long-term effectiveness as the immune system identifies it as foreign and eventually removes it. In the study NexCAR19, Dwivedi and her colleagues supplemented the mouse antibody tips with human proteins.
Laboratory experiments demonstrated that the ‘humanised’ CAR had similar antitumor efficacy as a CAR generated from mice while also causing a reduction in the production of cytokines, a kind of protein. Note that certain cancer patients undergoing CAR-T therapy may experience a severe inflammatory response known as cytokine-release syndrome, a potentially fatal condition.
Experimental data
Initial clinical trials with NexCAR19 in adult patients with various types of lymphoma and leukaemia demonstrated that out of the 33 individuals who had the treatment, 19 of them experienced total tumour regression at the one-month follow-up. The cancers in an additional four individuals had reduced in size by 50%, resulting in an overall response rate of 70%. We will monitor the trial participants for a minimum of five years.
“Only time will determine the future outcome of this situation,” says Hasmukh Jain, a medical oncologist at Tata Memorial Centre in Mumbai, who led the trials.
Natasha Kekre, a haematologist at the Ottawa Hospital, highlights that the findings are derived from a limited number of individuals with various types of blood malignancies, hence posing challenges in evaluating the effectiveness of the treatment for specific cancer types.
Out of all the patients, only two individuals had more intense manifestations of cytokine-release syndrome, but none of them experienced neurotoxicities, which is another typical but transient adverse effect of CAR-T therapy.
According to Kekre, the safety profile of this treatment is superior to that of certain FDA-approved CAR-T treatments. She attributes this to the product’s advancements and the scientific and medical community’s cumulative knowledge and experience over the years in enhancing patient care.
Rahul Purwar, an immunologist at the Indian Institute of Technology Bombay and founder of ImmunoACT, stated to the media that humanising the CAR likely played a role in the therapy’s favourable safety profile. However, some argue that we have not yet demonstrated the connection between the two.
Fry suggests that the specific environment and the characteristics of the patients receiving treatment in India may influence the outcomes. Various patient factors influence the toxicity profile of CAR-T cells.
Reducing expenses
Despite the fact that the cost of the treatment remains unaffordable for a significant number of Indians, whose yearly gross national income per person is below $2,500, the price of NexCAR19 provides optimism that CAR-T therapy can be produced at a lower cost in different nations and circumstances. In order to save expenses, the team successfully created, evaluated, and produced the product exclusively in India, taking advantage of the lower labour costs compared to wealthier nations.
Researchers commonly employ lentiviruses as a means of introducing CARs to T cells; however, this method can be costly. According to Steven Highfill, an immunologist at the US National Institutes of Health Clinical Centre in Bethesda, the cost of acquiring a sufficient amount of lentiviral vector for a trial involving 50 individuals can reach up to US$800,000 in the United States. Highfill has provided guidance to the Indian team. Scientists at ImmunoACT produce the gene-delivery vehicle in-house.
According to Highfill, the Indian team discovered a more cost-effective method to generate the modified cells in large quantities, eliminating the requirement for costly automated technology.
According to Purwar, the therapy’s enhanced safety profile compared to other FDA-approved medications significantly decreases patients’ expenses. Consequently, the majority of patients did not require prolonged stays in intensive-care units.
Purwar aims to achieve additional cost reductions, mostly by expanding production capacity. ImmunoACT intends to expand its market to Mexico by exporting its therapy. Additionally, the company aims to innovate by developing new medicines, namely a treatment for multiple myeloma, a different type of blood cancer.
However, ImmunoACT encounters competition. Several additional Indian firms have initiated local CAR-T studies, such as Immuneel Therapeutics in Bengaluru, which has obtained a licence for technology created by Spanish researchers.